Facility Output
+20%
Lot to Lot Variability
-50%
QA Investigations
-50%
Master Raw Material Quality and Achieve Unmatched Product Excellence
Our collaboration is dedicated to mastering raw material quality, ensuring that your products consistently meet the highest standards. By thoroughly analyzing variations in raw materials during both process development and GMP manufacturing, you are not just enhancing your understanding. You are building a resilient and robust process.
This proactive approach helps you reduce costs and conserve resources by preventing inconsistencies before they occur. Utilizing advanced high-throughput systems and DNA methylation analysis, you can accurately identify critical raw materials attributes early in the process.
Our Material Testing services are offered in two different depth levels to fit best your expectations.
Efficient Level
The Efficient Level service ensures minimal effort in the setup, qualification, and validation phases, ideal for small-scale operations processing approximately 10-15 batches annually.
Simplified Setup: The setup method requires only 12 samples, ensuring a swift and cost-effective initiation of your analytical processes.
Basic Qualification & Validation Included: This Level requires about 40 samples to generate accurate data, consistent performance and precise detection on specific parameters
Key Benefits
Expressing analysis report supporting informed decision-making.
Quick and straightforward method setup, qualification, and validation.
Highly efficient use of materials and resources.
Enhanced Level
The Enhanced Level service ensures a comprehensive analytical approach designed for customers with up to 50 batches per year and a higher need of method qualification and validation.
Setup Robustness: A total of 24 samples is processed for the method setup, providing accurate and reproducible results from the start.
Qualification & Validation Samples: This Level requires about 80 samples to generate precise data, consistent performance and accurate detection over a complete analytical range.
Key Benefits
Comprehensive analysis report supporting informed decision-making.
Enhanced regulatory confidence for your biotechnological cell culture operationsDeep analytical insights and increased reliability
The result? A detailed comparison report that highlights potential changes and their implications on product quality. Additionally, we enable you to develop and implement a robust quality control testing strategy that guarantees a stable, high-quality product throughout its entire lifecycle.
Together, we can ensure excellence in your products and foster a culture of quality that strengthens your commitment to innovation and reliability.
Enhanced Process Understanding
Identify critical raw material attributes and their impact on product quality.
Increased Robustness
Reduce inconsistencies and improve the overall output of manufacturing.
Cost Efficiency
Minimize expensive failures and reduce labor and time costs.

CMC Consulting
We ensure compliance with regulatory standards, optimizes product development, and enhances manufacturing processes, ultimately leading to high-quality, safe, and effective biopharmaceutical products.

Shortened Development Time
By predicting fed-batch experiment outcomes without performing them, we save time and costs, enhance process knowledge, and ensure robust, reproducible processes.

Scaling and Transfer Comparability
We’ve created a comparison report on potential changes and their impacts on product quality. Ultimately, it equips our clients with the tools to uphold high standards in bioprocessing.
Additional topics that may be of interest

Digital Twins & Prediction

Process Analytical Technology
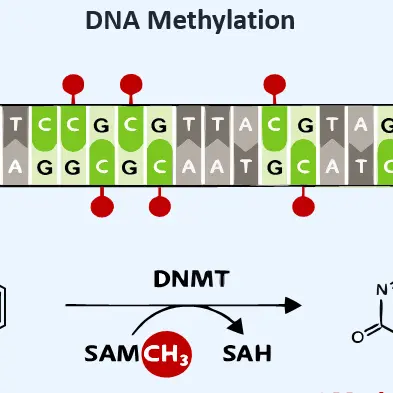
DNA Methylation in Bioprocessing

Qualification & Consulting
Let's Connect
Get in touch with your customers to provide them with better service. You can modify the form fields to gather more precise information.