Tox Studies
only once!
Time to Market
- 50%
Conformation Runs
- 3
We team up to uphold top-notch bioprocessing standards.
By using QBDC's advanced analytical technology, we improve comparability testing, which is essential for product quality and consistency.
By utilizing cells as sensors, we monitor processes and enable comparisons across different runs, sites, and process changes. Using QBDC's advanced analytical technology, we enhance comparability testing, which is crucial for maintaining product quality and consistency.
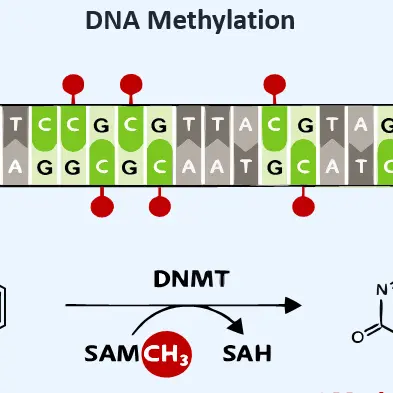
We analyze at least three data sets from the original process using microarray technology. This foundational work supports seamless technology transfer, allowing us to compare updated processes, assess changes by analysing the cells, and determine corrective actions. We also examine DNA methylation in cells from both original and transferred processes.
You receive a detailed comparison report outlining changes of cell DNA methylation and their effects on product quality. This facilitates quicker corrective actions and a deeper understanding of the process, leading to improved product quality. Ultimately, we provide biotech technicians with the insights they need to ensure high product standards and excel in bioprocessing.
Enhanced Process Understanding
Gain deeper insights into cell regulation and in the context of potential effects on product quality and bioprocess parameters
Improved Product
Quality
Ensure comparability and consistency in product quality across scaled or transferred processes
Efficient Corrective
Actions
Quickly identify and address process-related issues to maintain high product standards.

CMC Consulting
We ensure compliance with regulatory standards, optimizes product development, and enhances manufacturing processes, ultimately leading to high-quality, safe, and effective biopharmaceutical products.

Shortened Development Time
By predicting fed-batch experiment outcomes without performing them, we save time and costs, enhance process knowledge, and ensure robust, reproducible processes.

Control of Product and Raw Material Quality
We utilize high-throughput systems and DNA methylation analysis to define critical raw materials early. This proactive approach reduces costs and resources.
Additional topics that may be of interest

Digital Twins & Prediction

Process Analytical Technology
DNA Methylation in Bioprocessing

Qualification & Consulting
Let's Connect
Get in touch with your customers to provide them with better service. You can modify the form fields to gather more precise information.

